Contact Support
With the increasing risk of hazardous drug exposure in the veterinary practice, devices protecting healthcare workers have become a priority.
As stated in the ACVIM 2018 consensus, use of a closed system drug transfer device (CSTD) (such as BD Phaseal™) is recommended during preparation and administration of cytotoxic drugs. A CSTD mechanically prevents the escape of a drug or vapor out of the system into the environment.
To help prevent occupational exposure to hazardous drugs, BD offers a complete system of products that provide safe handling of hazardous drugs from end-to-end: drug mixing, preparation and delivery.
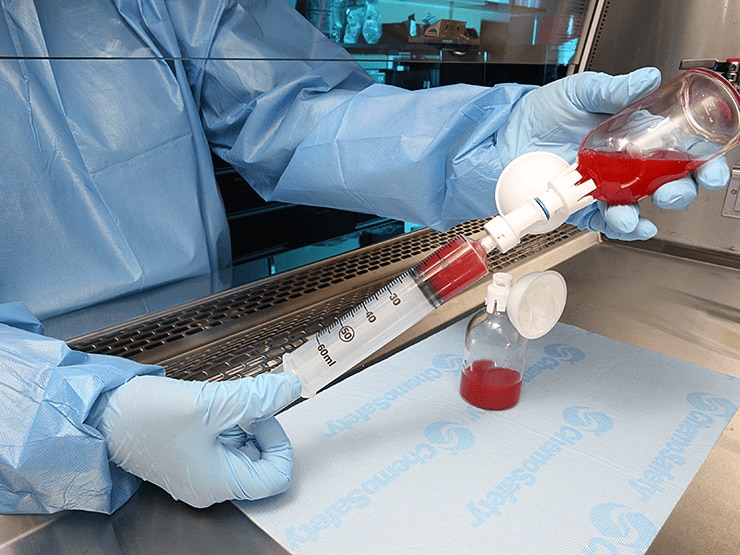
BD PhaSeal™ is clinically demonstrated to reduce personnel exposure to hazardous drugs. A protection for you and your staff when hazardous drugs are involved to avoid potential health risk with even limited or infrequent exposure.
Hazardous Drug Safety catalogue